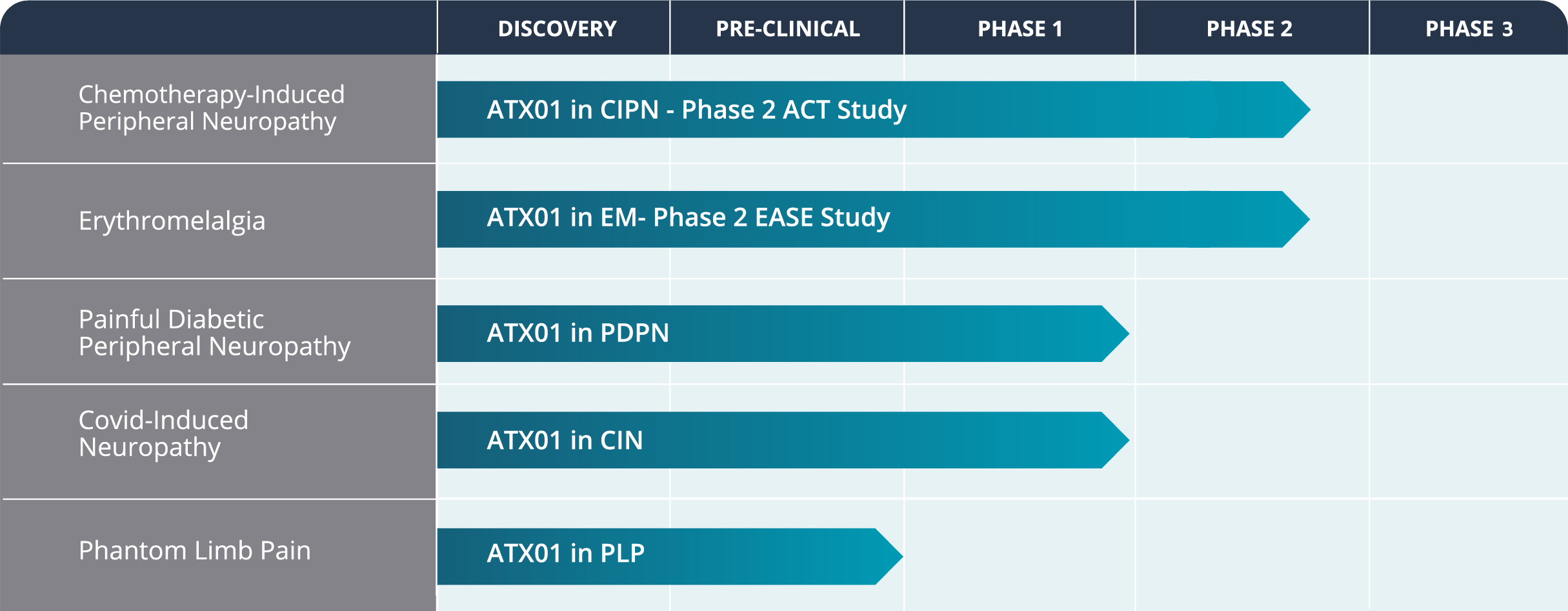
Our lead asset, ATX01, targets the signaling pathways involved in the regulation of pain by inhibiting specific nociceptive sodium channels.
It has been designed to provide targeted relief from excruciating pain in the feet and hands of patients suffering from Chemotherapy-Induced Peripheral Neuropathy (CIPN), a common and painful attack of cancer chemotherapy on peripheral nerve fibers.
AlgoTx’s ATX01 product has wide potential applicability beyond CIPN, in other diseases where peripheral neuropathic pain is a major issue.
By applying the ATX01 hydrogel to the skin, the active ingredient is locally delivered to the nerve endings where pain signals originate and propagate alongside the neurons to the central nervous system. This method of application is especially relevant to CIPN which involves localized pain. Topical administration also minimizes systemic toxicity and drug interactions through limited systemic exposure, which is particularly important in patients exposed to chemotherapy.

Unmet Need
Our lead asset, ATX01, has been designed to provide targeted relief in CIPN.
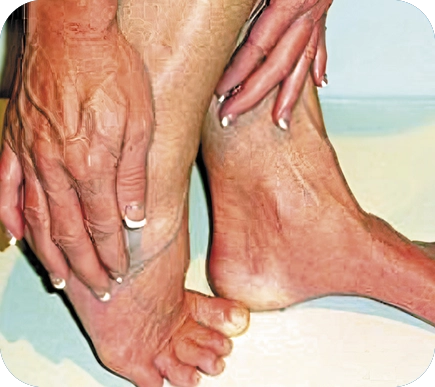
CIPN occurs in 68% of patients on chemotherapy and results from chemotherapy causing damage to the patient’s pain-signalling nerve fibers. It can be crippling, with chronic burning and stabbing pains in the feet and/or hands, as well as allodynia – feeling pain from actions that are not normally painful.
CIPN pain significantly influences quality of life and prevents patients from performing their daily personal and professional activities. Furthermore, it can be strong enough to cause cessation or alterations of cancer treatments, which in turn
impact cancer-related morbidity and mortality.1&2
Around 30% of patients will still be suffering CIPN a year or more after finishing chemotherapy. Neither preventative nor
symptomatic therapies have shown significant clinical efficacy.3 Treating the pain of CIPN represents a considerable unmet
medical need.4
AlgoTx is recruiting 240 patients into a pivotal Phase II clinical trial in CIPN ( the ‘ACT’ study) conducted in the USA and 6 European countries. This trial is due to provide its final data read out in H2 2024, and the company is making plans to expedite a Phase 3 trial and registration.
ATX01 was granted FDA fast track development status in CIPN.
For details of AlgoTx’s expanded access policy for CIPN, click here:

Unmet Need
The pharmacological profile of ATX01 makes it relevant to test in Erythromelalgia (EM).
EM affects 1 to 2 in 100,000 people. There are around 5,000 EM patients in the US and around 7,000 in Europe. This condition primarily affects the patient’s feet and hands, and less commonly, arms, legs, ears, and face. The condition presents as episodes of intense, burning pain, increased skin temperature and severe redness (erythema), that may last from minutes to days. To date, no treatment has proven its ability to meaningfully relieve the pain of EM.
AlgoTx has successfully recruited 13 EM patients into a Phase II trial run under IND at the Mayo Clinic (Rochester, Ma, US) and at the Erlangen University Hospital (Germany) under Fast-Track designation from the FDA.
AlgoTx successfully applied to FDA and EMA for Orphan Drug Designation, which provides additional regulatory support and enhanced market exclusivity once a new medicine has completed clinical trials and has secured market authorization.
For details of AlgoTx’s extpanded access policy for EM, click here:


Following the completion of the CIPN program, AlgoTx plans to develop ATX01 in additional indications where peripheral neuropathic pain is a major issue, such as Painful Diabetic Peripheral Neuropathy and Phantom Limb Pain (Pre-IND).